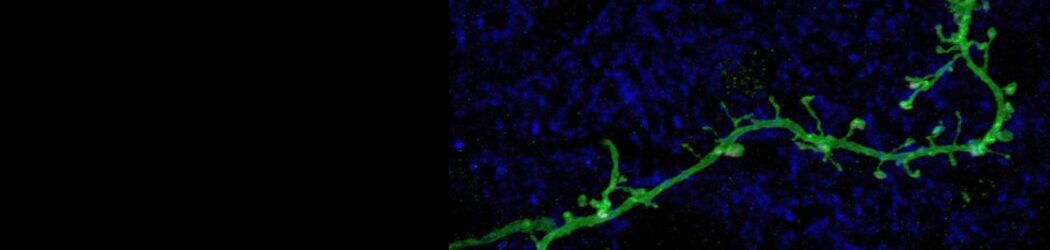
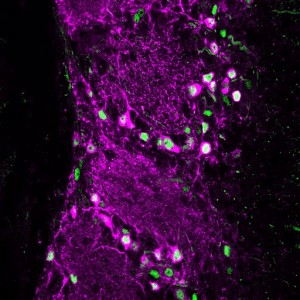
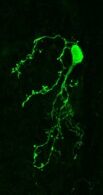
Your brain contains billions of inter-connected cells, or ‘neurons’, which work by sending electrical signals from one to another. These electrical events allow your nervous system to sense, to think, and to control your body. They can also change the way the brain works. In a process called ‘plasticity’, the past history of electrical activity in a neuron can alter its shape, its function, and the way in which it uses genetic information encoded in its DNA. Neuronal plasticity is a lifelong process, but it is especially prominent when cells are young, or after disruptive events such as nervous system injury. Our lab studies plasticity so that we can understand how our experiences can influence the way our brains develop, how they change over time, and how they can recover from damage.
To do this, we investigate neuronal plasticity in a part of the brain called the ‘olfactory bulb’. This is the first brain region to receive information about smells from the nose. It processes that sensory information and then passes it onto other parts of the brain where our perceptions of smell and flavour are created. We’re interested in the olfactory bulb because we’re fascinated by how our sense of smell works, and also because it has some unusual properties which make it a great model system to study many processes of neuronal plasticity.
Indeed, the olfactory bulb is an especially plastic part of the brain, meaning that it changes a lot depending on its recent history of activity. And even by the standards of the bulb, one specialised type of neuron found there is especially plastic. ‘Dopaminergic’ cells of the olfactory bulb are a local population of neurons that, by releasing the neurotransmitter dopamine (hence the name!), regulate the processing of olfactory information at its very earliest stages. Our recent work has shown that these dopaminergic cells change in many ways after even pretty brief alterations to the sensory environment – for example, after a nostril blockage for just one day, just like everyone often experiences during a common cold. We have also found that these dopaminergic cells are not all the same. Despite all using dopamine as a neurotransmitter, some of them have very different properties, and different forms of plasticity, to others. We are currently studying these differences to try to understand exactly how such variability – between cells that superficially look very similar – might actually lead to distinct functional roles in sensory processing.
Dopaminergic cells in the olfactory bulb are also plastic to the point that they completely replace themselves in a process known as ‘adult neurogenesis’. Whereas most of the neurons in the mammalian brain are generated in the womb, and can’t be replaced if they’re lost, certain types of olfactory bulb cells – including some of the dopaminergic ones – continue to be freshly-generated throughout life. We think this is amazing, and our research aims to find out how individual adult-born dopaminergic cells in the olfactory bulb can change themselves over time, responding to changes in the environment to regulate the way they contribute to sensory function.
To add just a little more complexity, the olfactory bulb networks that contain constantly regenerating dopaminergic neurons also receive most of their incoming information from a population of cells that are themselves continually regenerating. These cells – the ‘sensory neurons’ in the nose that do the initial work of detecting smell signals – are incredibly good at renewing themselves, and unlike most parts of the nervous system are capable of decent levels of recovery after injury or infection. We are currently working on understanding how the regeneration of these input neurons impacts on circuits in their target structure – the olfactory bulb. Are there changes in olfactory bulb neurons that help or hinder the functional regeneration of their input partners, and which might shape the ability of an animal to successfully recover its sense of smell?
These are all basic scientific questions, but one day they could have serious clinical relevance. By studying plasticity in the olfactory bulb, we hope to stimulate research into novel treatments for smell disorders – a serious set of conditions with huge impact on people’s health and quality of life, and which affect a surprisingly large proportion of the population. Much more broadly, by investigating the mechanisms and consequences of adult neurogenesis, we hope to one day inform treatment strategies trying to replace human cells lost through injury or disease. If we’re trying to replace old cells with new cells, why not take a few hints from the way the brain does it all by itself? Finally, by studying olfactory bulb plasticity during naturally occurring sensory neuron regeneration, maybe we can co-opt some of the same general strategies to improve functional recovery after damage to other parts of the nervous system which themselves cannot usually regenerate. For example, perhaps the olfactory bulb might be able to teach the post-injury spinal cord a thing or two about how to successfully welcome and make the most of newly grown inputs?