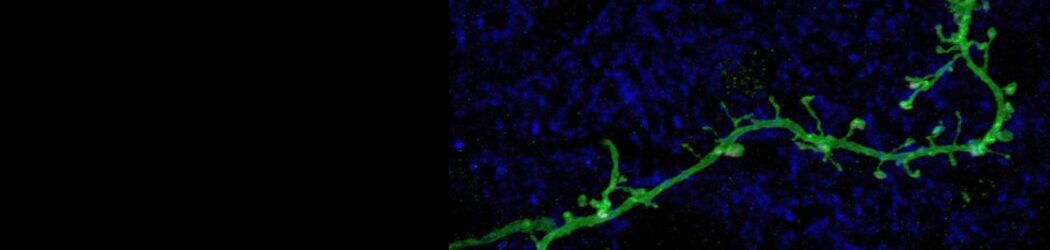
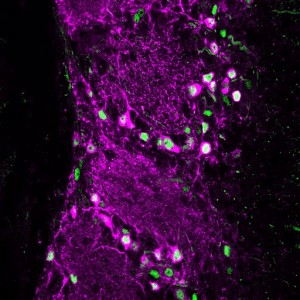
Our lab focuses on neuronal plasticity and the ways in which it shapes the brain’s development, its function, and its regeneration after injury. To do this, we study the activity-dependent maturation of circuits in the olfactory bulb (OB), asking how this part of the brain responds to various plasticity-inducing changes in its sensory inputs.
The olfactory bulb
The olfactory bulb (OB) is the first region of the brain to process smell information arriving from the nose, and is a fantastic model system for studying activity-dependent neuronal plasticity. Not only are OB circuits set up in an activity-dependent manner during early postnatal development (Tufo et al. 2022; Dorrego-Rivas & Grubb 2022), they are also continually altered throughout life as a consequence of cellular plasticity and ongoing adult neurogenesis. In addition, the olfactory system has unique regenerative capabilities, allowing us to study the ways that neuronal circuits can change during naturally occurring recovery from nervous system injury.
Olfactory bulb dopaminergic neurons
Our work focuses particularly on the bulb’s glomerular layer, where incoming sensory inputs are extensively modulated by local circuitry before being relayed to olfactory cortex. Within this glomerular network is a sizeable population of dopaminergic interneurons that co-release both GABA and dopamine to regulate transmission of olfactory information to bulbar projection neurons. These dopaminergic neurons are known to be extremely plastic; for example, they have exquisite activity-dependent control over expression of their transmitter synthesising enzymes. They are even plastic to the point of whole-neuron replacement, forming part of the OB interneuron population continually turned over throughout life via adult neurogenesis. But how do cellular mechanisms of plasticity interact with postnatal neurogenesis to enable DA neurons to control sensory processing at the first stages of olfaction?
Heterogeneity of olfactory bulb dopaminergic neurons
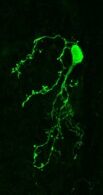
Our initial work on this key question started by uncovering significant heterogeneity amongst this initially
Two major subtypes of dopaminergic neuron in the OB: one without an axon, one with! (Galliano et al. 2018)
homogenous-seeming OB dopaminergic population. We identified two distinct dopaminergic subtypes. One subtype behaves just like the ‘canonical’ neurons found in most areas of the mammalian brain, with exclusively embryonic generation, and separate axonal and dendritic domains. The other subtype would be very unusual anywhere but the OB – its lack of an axon and its ongoing neurogenesis throughout adult life are not features normally associated with mature neurons, but are actually pretty standard for OB inhibitory interneurons (Galliano et al. 2018). Ongoing work in the lab is now centred on understanding variability in neuronal polarity, gene expression, electrophysiological function and network connectivity within this diverse population of dopaminergic cells. To do this, we are using single-cell RNAseq (Brann et al. 2020), Patch-seq (Lipvsek et al. 2020) and smFISH approaches, together with acute slice patch-clamp recordings and morphological analysis.
Activity-dependent plasticity of dopaminergic subtypes
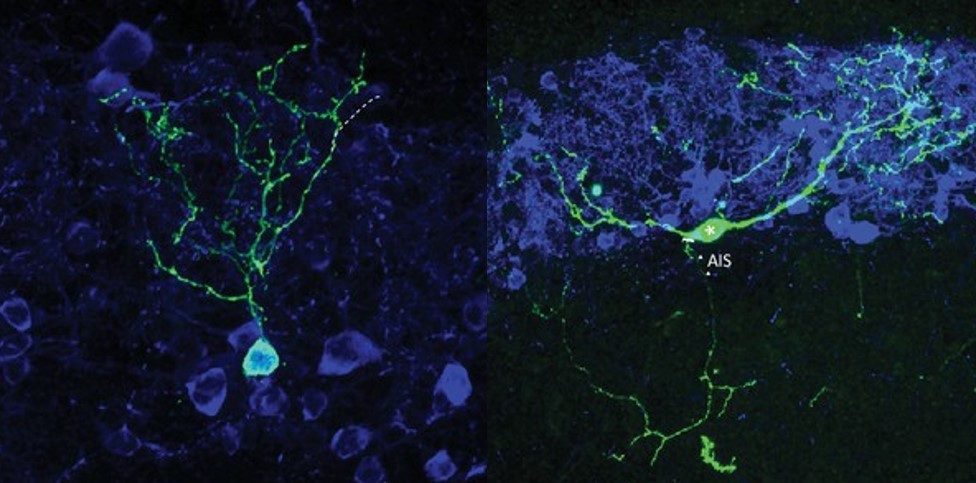
With a clearer picture of heterogeneity among dopaminergic cells in our model system, we have been able to focus our attention on the plastic changes induced in these two types of OB dopaminergic cell (as well as other key components of the glomerular network) by controlled manipulations of sensory activity in vivo. We have identified cell-type and cell-subtype-specific forms of structural and functional plasticity
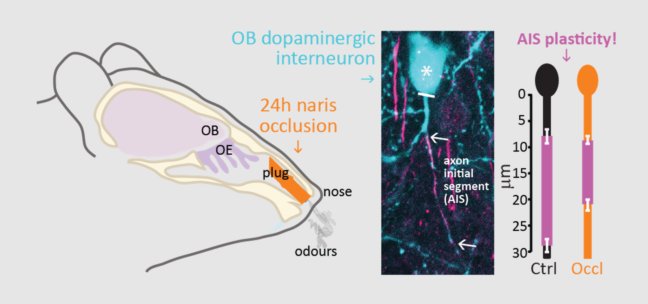
AIS plasticity in OB dopaminergic neurons (Galliano et al. 2021)
in OB circuits following a brief, physiologically relevant 1-day manipulation of sensory experience (Galliano et al. 2021; Byrne et al. 2022). These include plastic changes at the axon initial segment (AIS) of axon-bearing dopaminergic neurons (Galliano et al. 2021) – the first evidence for experience-driven AIS alterations in interneurons in vivo, built on a foundation of work we established for the forms and mechanisms of AIS plasticity in vitro (Grubb & Burrone 2010; Evans et al. 2013, 2015, 2017; Chand et al. 2015; Dumitrescu et al. 2016). We are now using our knowledge of dopaminergic heterogeneity to study how experience-dependent plastic changes in gene expression in this cell population might influence neuronal physiology and sensory-processing.
Adult neurogenesis
We are also investigating how these experience-dependent plastic responses in OB neurons interact with ongoing neurogenesis under different conditions. Is plasticity especially strong in immature adult-born dopaminergic neurons, and if so, how does this contribute to the sensory-processing functions of the glomerular network?
Naturally occurring regeneration
Last, but by no means least, we are increasingly interested in how OB plasticity can influence recovery from nervous system injury. Olfactory sensory neurons in the nose are remarkably capable of regeneration after damage caused by infection, inflammation, toxins or physical insult. Once regenerated, they grow axons back to the brain, re-establishing functional connectivity with OB circuits, and recovering the ability to drive sensory-guided behaviour. Using this unique model of naturally occurring regeneration in the adult mammalian brain, we are currently focused on understanding how plasticity in central OB neurons can shape this functional recovery. Our first findings established that re-connecting sensory axons have almost fully mature presynaptic properties as soon as they form new synapses with their postsynaptic OB partners, and that full maturation of these presynaptic properties occurs in just a few days (Browne et al. 2022). We are now asking how structural, functional and transcriptomic alterations in different OB cell types are produced by degeneration and subsequent regeneration of the circuit’s driving sensory inputs, and how these plastic changes can contribute to functional recovery after injury. Ultimately our hope is that if we can gain sufficient understanding of plasticity in the brain under natural circumstances, we might be able to harness this understanding to promote recovery of any CNS region after injury or in disease.
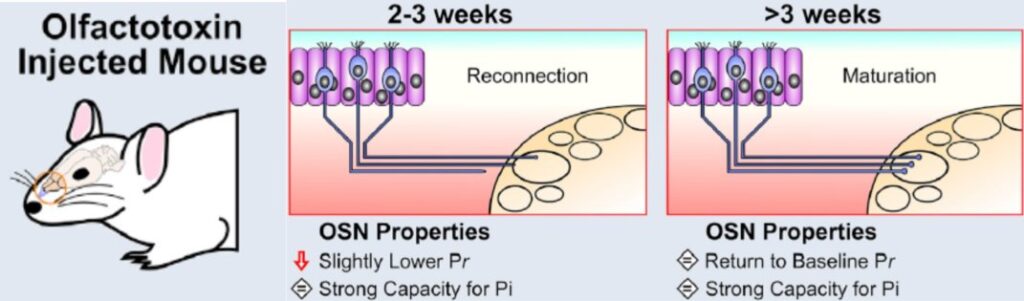
Rapid maturation of naturally regenerating axon terminals (Browne et al. 2022)